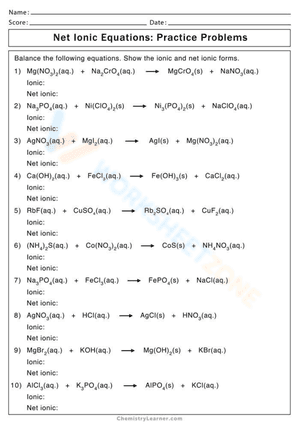
Only the chemicals directly engaged in the chemical reaction are shown in the chemical equation known as the net ionic equation. The positively charged silver cation was listed first on the reactant side of the net ionic equation, followed by the negatively charged chloride anion. Because that is the sequence in which the ions must be recorded in the silver chloride product, this is rather customary. The order of the reactants does not necessarily have to be this way, though. In net ionic equations, mass and charge must be equal. If you are struggling with problems concerning net ionic equations, please don’t be worried. On this website, we have prepared numerous net ionic equation worksheets for extra practice. These Chemistry worksheets will give students a chance to practice a variety of problems and activities to help students dive deeper into the topic. Try our net ionic equation worksheet collection if you're seeking a way to reteach and offer further help when it comes to this topic. We’re sure that it would be an excellent reinforcement resource.
Periodic TableBalancing ActConservation Of MassClassifying MatterLab SafetyChemical Bonding Ionic And CovalentAcid NamingBalancing Chemical EquationsHesss LawColligative PropertiesLe Chatelier's PrincipleNaming Molecular CompoundsMole RatioNova Hunting The ElementsCalculating Average Atomic MassEndothermic And Exothermic ReactionNaming Ionic CompoundsStoichiometryMetals Nonmetals And MetalloidsNaming AlkanesMixtures And SolutionsMole To Grams Grams To Moles ConversionsAtoms Isotopes And IonsEmpirical/Molecular Formula PracticePredicting Products Of Chemical ReactionsSolubility CurveIntermolecular ForcesProtons Neutrons And Electrons PracticeIsotope PracticeNaming Covalent CompoundsPercent CompositionTypes Of ReactionClassifying Chemical ReactionsConjugate Acid Base PairsDalton's Law Of Partial PressureGrahams LawPeriodic TrendsCounting AtomsAssigning Oxidation NumbersSingle Replacement ReactionPh And Poh CalculationsLab EquipmentLimiting ReactantNeutralization ReactionsMolar MassElectron Configuration PracticeHistory Of An AtomBohr ModelIntegrated Science CyclesPhotosynthesisOn Nitrogen CycleMacromoleculeBiogeochemical CyclesBalanced And Unbalanced ForcesKaryotypeProkaryote And Eukaryote CellsThe Carbon CycleInsulin To Carb Ratio
Allfiltered results