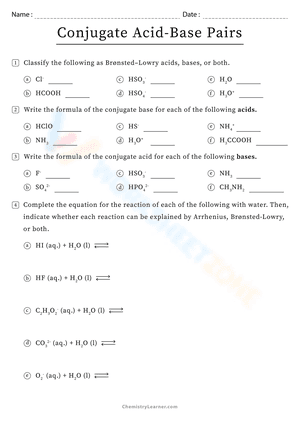
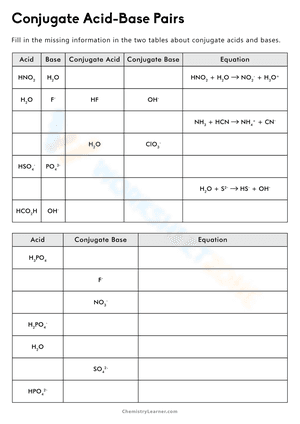
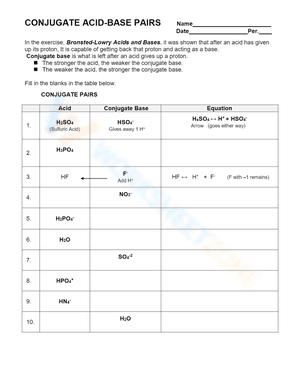
Acids and bases exist in the form of conjugate acid-base pairs. The word conjugate, which means "joined together" in Latin, describes objects that are connected, especially in pairs, like Bronsted acids and bases. A conjugate base is created every time a Bronsted acid acts as an H+-ion donor. We may simply state the relative strengths of acids and bases by using conjugate acid-base pairs. The stronger a base, the weaker its conjugate acid, and the stronger an acid, the stronger its conjugate base. If you are struggling with problems concerning conjugate acid-base pairs, please don’t be worried, we have prepared dozens of conjugate acid-base pairs worksheets for extra practice. These Chemistry worksheets will give students a chance to practice identifying acids, bases, and their conjugate pairs. There will also be different problems and activities such as writing acid-base equations or identifying which substance is donating and which is accepting the proton. If you're looking for a way to reteach and provide further assistance when it comes to this issue, give these conjugate acid-base pairs worksheets a try. We’re sure that it would be a great reinforcement resource.
Periodic TableBalancing ActConservation Of MassClassifying MatterLab SafetyChemical Bonding Ionic And CovalentAcid NamingBalancing Chemical EquationsHesss LawColligative PropertiesNet Ionic EquationLe Chatelier's PrincipleNaming Molecular CompoundsMole RatioNova Hunting The ElementsCalculating Average Atomic MassEndothermic And Exothermic ReactionNaming Ionic CompoundsStoichiometryMetals Nonmetals And MetalloidsNaming AlkanesMixtures And SolutionsMole To Grams Grams To Moles ConversionsAtoms Isotopes And IonsEmpirical/Molecular Formula PracticePredicting Products Of Chemical ReactionsSolubility CurveIntermolecular ForcesProtons Neutrons And Electrons PracticeIsotope PracticeNaming Covalent CompoundsPercent CompositionTypes Of ReactionClassifying Chemical ReactionsDalton's Law Of Partial PressureGrahams LawPeriodic TrendsCounting AtomsAssigning Oxidation NumbersSingle Replacement ReactionPh And Poh CalculationsLab EquipmentLimiting ReactantNeutralization ReactionsMolar MassElectron Configuration PracticeHistory Of An AtomBohr ModelIntegrated Science CyclesPhotosynthesisOn Nitrogen CycleMacromoleculeBiogeochemical CyclesBalanced And Unbalanced ForcesKaryotypeProkaryote And Eukaryote CellsThe Carbon CycleInsulin To Carb Ratio
Allfiltered results