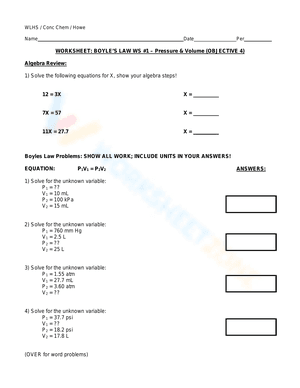
The inverse relationship between the pressure and volume of a container of gas held at a constant temperature is explained by Boyle's law. It was first discovered by Richard Towneley and Henry Power in the 17th century; and a few years later, Robert Boyle finally confirmed and published it. Boyle's law can be expressed mathematically as PV=k, where P is the pressure measurement, V is Volume, and k is a constant. It states that the pressure and volume, when multiplied together, will remain constant as long as the temperature and mass remain constant. With our Boyles Law worksheets, you will be given a chance to practice using the formula PV = constant to calculate the pressure or volume of a gas that is permitted to expand or contract at a constant temperature. It will also contain questions that require students to use their understanding of Boyle's Law to address issues with the pressure and volume of a fixed mass of gas at a fixed temperature. If you are looking for a way to re-teach and provide additional practice with this law, give our Boyles Law worksheet a try. We’re sure that these Chemistry worksheets would be a great reinforcement activity.
Worksheets
Quizzes
Lesson Plans
Sort by
Popular
Allfiltered results
By Grade
By Activity
Related Searches
Charles' Law
Pressure-Volume Relationship
Ideal Gas Law
Gas Constant